- Home
- Live Blog
- Breaking News
- Top Headlines
- Cities
- NE News
- Sentinel Media
- Sports
- Education
- Jobs

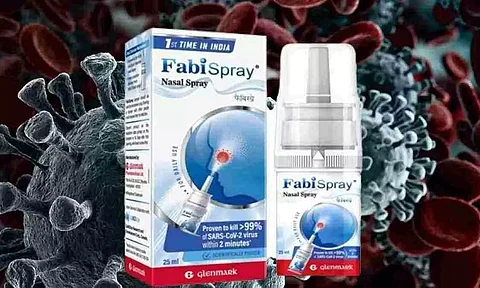
NEW DELHI: Indian pharmaceutical company in Mumbai, Glenmark Pharmaceutical Limited has launched the first nasal spray in the country called FabiSpray- Nitric Oxide Nasal Spray (NONS) to treat adult COVID-19 patients.
The Nitric Oxide nasal spray got the confirmation of destroying 99.9 percent of severe acute respiratory syndrome coronavirus 2 including Alpha, Beta, Delta and Gamma in 2 minutes according to the studies in the Utah State University of USA.
The worldwide business running company Glenmark has acquired the marketing and manufacturing authorization for FabiSpray from the Drug Controller General of India which is drug the authority of India. It was a part of the quick approval process for the first nasal spray.
In an official statement, it was informed that the main aim of the third phase study in India was completed with viral load reductions of 94% in 24 hours and 99% in 48 hours.
The statement also reads that the Nitric Oxide Nasal Spray worked well in the covid positive patients and it was completely safe without any side effects. So the firm has decided to offer the nasal spray which will be available under the brand name FabiSpray.
Glenmark further informed that at the time when the NONS was sprayed over nasal mucosa to perform the experiment it was found that the nasal spray works as a chemical and physical hindrance against the covid virus.
The spray also prevents the virus from incubating and stops the deadly virus from getting extended to the lungs of a person. It consists of anti-microbial belongings with a direct virucidal impact on SARS-CoV-2 (severe acute respiratory syndrome coronavirus 2).
The chief commercial officer of Glenmark, Robert Crockart said that the company is confident about the nasal spray functioning well and said that it will be helpful for patients in offering a timely and much-needed therapy.
He also said that as a leading pharmaceuticals company, Glenmark is a crucial part in fighting the deadly coronavirus in the country and therefore it has obtained the regulatory clearance for India's first nasal spray.
The head of the clinical Dept. and Senior VP of the company, Dr. Monika Tandon said that the outcomes of the NONS from the third phase, placebo-controlled test and double-blind are promising.
Glenmark has launched FabiSpray in collaboration with SaNOtize for treating those adult patients affected with the COVID-19 and suffering with the virus.
Also watch: