- Home
- Live Blog
- Breaking News
- Top Headlines
- Cities
- NE News
- Sentinel Media
- Sports
- Education
- Jobs

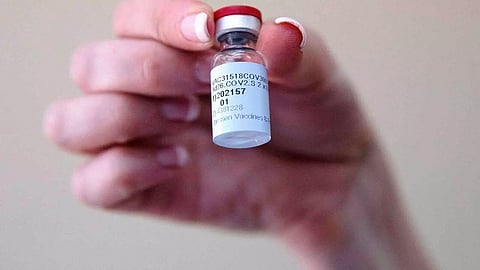
NEW YORK: The US is on the cusp of getting a third vaccine against COVID-19. The US Food and Drug Administration today delivered a strong endorsement of Johnson & Johnson's single-dose vaccine and set the stage for a final decision on February 26.
Two vaccines - from Pfizer and Moderna - have already received emergency use approval in the US. US FDA scientists confirmed that the Johnson & Johnson vaccine is safe and is about 66 per cent effective at preventing moderate to severe COVID-19, and about 85 percent effective against serious illness.
On February 26, a special team of independent US FDA advisors will debate if the shots can be recommended for population level use - following the same greenlighting process for the two earlier vaccines.
The COVID-19 death toll in the US has topped 500,000 but the country's infection curve is slowly bending downwards. More than 44.5 million Americans have received at least one dose of vaccine made by Pfizer or Moderna, and nearly 20 million of them have received the second dose.
J&J tested its single-dose option in about 44,000 adults in the US, Latin America and South Africa with a 2-month median follow-up. "The analysis supported a favorable safety profile with no specific safety concerns identified that would preclude issuance of an EUA," the US FDA said of the J&J vaccine.
The FDA summary noted that there were no COVID-19-related deaths and no COVID-19 cases requiring medical intervention 28 days or more post-vaccination among participants age 60 years or older with medical comorbidities in the vaccine group. (IANS)