- Home
- Live Blog
- Breaking News
- Top Headlines
- Cities
- NE News
- Sentinel Media
- Sports
- Education
- Jobs

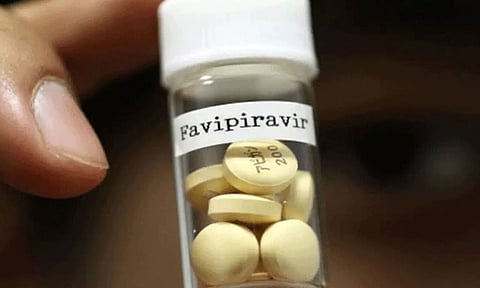
Guwahati: Mumbai-based Glenmark Pharmaceuticals on Saturday said it has launched FabiFlu, containing the antiviral drug Favipiravir, for the treatment of patients with mild to moderate Covid-19.
The Drugs Controller General of India (DCGI) had given manufacturing and marketing approval to the drug firm on Friday.
The company said in a statement that the FabiFlu is the first oral Favipiravir-approved medication for the treatment of the viral disease.
Reacting to the development, Glenn Saldanha, Glenmark Pharmaceuticals Chairman, and Managing Director, said that this approval comes at a time when cases in India are spiralling like never before, putting tremendous pressure on the nation's healthcare system.
He added that the company hopes that the availability of an effective treatment such as FabiFlu will considerably help assuage this pressure, and offer patients in India a much needed and timely therapy option.
This development has come days after dexamethasone, a commonly used steroid that is currently used to reduce inflammation in other diseases, has become the first drug shown to be able to save Covid-19 patients.
Glenmark Pharmaceuticals is a pharmaceutical company headquartered in Mumbai, India that was founded in 1977 by Gracias Saldanha as a generic drug and active pharmaceutical ingredient manufacturer; he named the company after his two sons. The company initially sold its products in India, Russia, and Africa, but has expanded its network to more nations around the world.