- Home
- Live Blog
- Breaking News
- Top Headlines
- Cities
- NE News
- Sentinel Media
- Sports
- Education
- Jobs

New Delhi:
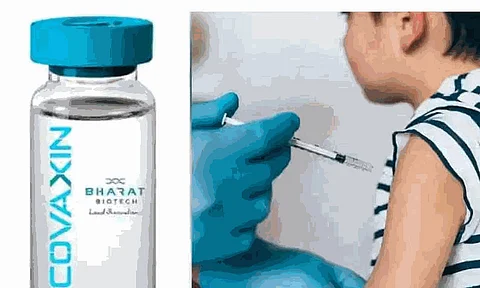
According to reports, screening of children for clinical trials of Covaxin will begin today at AIIMS in Delhi, only days after similar studies began at AIIMS in Patna.
The All India Institute of Medical Science (AIIMS) in New Delhi will perform clinical trials of the COVID-19 vaccine developed by Bharat Biotech to evaluate if it is safe for children aged two to eighteen.
Experts have cautioned that if enough individuals are not immunized against the COVID-19 virus, a subsequent breakout of the third wave might create as much damage as the second wave and that children might be its major target.
The vaccines used in India, however – Covaxin, Covishield, and Sputnik V – are not authorized for use on children.
Last month, Dr. VK Paul, a senior member of the NITI Aayog, indicated that the phase 2/3 trials will be conducted on children aged two to eighteen. On May 13, the centre authorised the clinical trials.
Also Read [COVID-19 vaccine safe during pregnancy']
The vaccine developed by Pfizer-BioNTech has been approved for use in certain age groups of children in the United States and Canada. China has also approved the use of CoronaVac, a COVID-19 vaccine developed by the Chinese firm Sinovac, as an emergency treatment for children aged 3 to 17 years. Most nations, however, are not currently immunising youngsters against the virus.
The second wave of the novel coronavirus, which hit India in March, has brought the country's healthcare infrastructure to its knees, killing hundreds of people on a daily basis. Many COVID-19 patients have died as a result of a lack of medical oxygen, hospital beds, and crucial life-saving medications.
However, the situation is gradually stabilizing as the Covid wave begins to recede. India, which had nearly 4 lakh daily cases in April, recorded 1.14 lakh new cases on Sunday, the lowest in two months.
Keeping in mind the possible outbreak of the third wave of the novel coronavirus pandemic, many Indian states in order to prepare themselves for the potential outbreak have been extensively setting up special Pediatric intensive care units and special healthcare centres for children.